中文摘要
淡水资源是人类赖以生存的基本物质.但是由于生活工业污水的排放,环境污染日益严重,淡水资源急剧短缺.所以解决可利用水资源的问题至关重要.众所周知,海水资源占据地球上水资源的绝大部分,但是由于海水中含有大量的氯化钠、氯化镁等盐类,并不能被直接利用,需要对其进行淡化后才能使用.其中,电容去离子技术(CDI)由于其具有淡化效果好、成本低和综合效益好的特点被广泛关注.电极是电容去离子中的核心部分,它的性能极大的影响了淡化性能.在选择电极材料时,为保证淡化能力,应选用来源广泛,成本较低的材料.本论文中选用了椰壳和钠锰氧化物作为原材料制备电极.
论文将椰壳通过马弗炉煅烧和化学处理后得到了椰壳炭,并将椰壳炭与其他材料一起辊压成片电极.将制备成的电极以CDI模块测试系统对其脱盐性能进行测试研究.溶液的电导率随时间变化,说明CDI模块能够成功的将待测溶液中的盐去除和脱附.在不同盐溶液流速情况下,溶液电导率首先下降较快,接着逐渐趋于平缓.电导率随着溶液流速的增加下降较快,CDI效果逐渐变好,但是继续增加溶液的流速至35r时,CDI效果变差.综合能耗与效益最大化,30r(10mL/min)的流速为最优选择.在不同操作电压下,操作电压越大,溶液中电导值下降幅度大.所研究的电压中电压为1V时吸附效果最好,电导率最低降至950μS/cm.将充放电仪反接后,脱附后得到的溶液最大电导值与最初始的溶液电导值基本一致,这表明所制备的椰壳炭材料电极同时也具备有良好的脱附性能.在处理不同盐溶液时,对不同离子电荷量和离子半径的吸附情况不同.不同离子吸附规律为电荷高吸附量大,所带电荷一致时,半径小吸附量较高.
论文使用钠锰氧化物(NMO)作为原材料,采用固相反应来合成制备了Na4Mn9O18,与其他材料混合均匀后辊压机压延成片型电极.以同样的方法制备了活性炭(Activcatedcarbon)电极.将NMO电极和AC电极组装成非对称系统.使用循环伏安法对NMO电极和AC电极测试,两种电极在水溶液中表现出优良的稳定性和高容量.同时对 NMO/AC 非对称系统除盐效果进行测试.最大的离 子移除速率可以达到 0.066 mg(NaCl)/g/s.与 CDI 系统(0.048 mg/g/s)使用对称 活性炭电极所得出的结果相比,性能较好.在处理较高浓度的盐溶液时也具备较 好的处理效果.Na4Mn9O18 具有较大的充电容量(40 mA h/g~50 mA h/g),并 且该值超过 300 F/g,比 AC 材料有更高的电容.同时在循环期间,充/放电容量 显示出极好的稳定性,300 圈循环后库仑效率接近 100%.说明 NMO/AC 非对称 系统具有良好的电化学性能,同时在氯化钠水溶液中可多次循环使用.
关键词:电容去离子,电极,海水淡化,离子
Abstract
Fresh water is the basic material for human survival. However, due to the discharge of industrial sewage, the environmental pollution is becoming more and more serious, and the fresh water resources are in short supply. So it is very important to solve the problem of using water resources. As we all know, sea water resources occupy the vast majority of water resources on the earth. However, because seawater contains a large number of salts such as sodium chloride and magnesium chloride, it can not be directly used, so it needs to be desalinated before it can be used. Among them, capacitive deionized Technology (CDI) has been widely concerned because of its advantages of good desalination, low cost and good comprehensive benefits. The electrode is core part of the capacitive deionization, and its performance greatly affects the desalination performance. While selecting electrode materials to ensure desalination, a wide range of materials with low cost should be selected. In this paper, coconut shells and sodium manganese oxides are selected asthe raw materialsfor the preparation of the electrode.
The coconut shell was treated by muffle furnace and chemical procedures and coconut shell activated carbon was obtained. The coconut shell charcoal together with other materials were roled into electrode pieces. The desalting performance of the prepared electrode was tested by the CDI module test system. The electrical conductivity of the solution varies with time, indicating that the CDI module can successfully remove and deactivate the salt in the solution. At the flow rate of different salt solutions, the conductivity of the solution first descends faster and then gradually tends to slow. With the increase of the flow velocity of the solution, the effect of CDI gradually becomes better, but the effect of CDI becomes worse when the flow rate of the solution increases to 35 r. The maximum energy consumption and efficiency are maximized, and the flow velocity of 30 r (10 mL/min) is the best choice. Under the different operating voltages, the greater the operating voltage, the decrease of the conductivity in the solution. When the voltage in the voltage is 1V, the adsorption effect is the best and the conductivity is lower to 950 μS/cm. The charging and discharging device will reverse, the maximum conductivity solution desorption values obtained with the initial conductivity values are consistent, which indicate that the coconut shell carbon electrode preparation also has good desorption performance. The adsorption of different ionic charge and ion radius is different when different salt solutions are treated. The adsorption of different ions is high in the high charge of charge. When the charge is consistent, the small adsorption capacity is higher.
Using sodium manganese oxide (NMO) as raw material, solid phase reaction is used to synthesize Na4Mn9O18, mixed with other materials, roll press is rolled into sheet type electrode. The AC electrode was prepared by the same method. The NMO electrode and the AC electrode were assembled into asymmetric systems. NMO electrode and AC electrode were tested by cyclic voltammetry. The two electrodes showed excellent stability and high capacity in aqueous solution. At the same time, the desalting effect of NMO/AC asymmetric system was tested. The maximum removal rate of ion can reach 0.066 mg (NaCl)/g/s. Compared with the results obtained from the CDI system (0.048 mg/g/s) using a symmetrical activated carbon electrode, the performance is better. It also has good treatment effect when treating high concentration of salt solution. Na4Mn9O18 has a larger charging capacity (40 mA h/g - 50 mA h/g), and the value is over 300 F/g, and has a higher capacitance than the AC material. At the same time, the charge/discharge capacity shows excellent stability during the cycle, and the coulomb efficiency is close to 100% after 300 cycles. This indicates that the electrochemical performance of the NMO/AC asymmetric system is stable and can be used repeatedly in the sodium chloride solution.
Key word: Capacitive; deionization; electrode; sea water; desalination ion
目 录
第 1 章 绪 论......................................................................................... 1
1.1 前言.................................................................................................. 1
1.2 CDI 的历史背景.............................................................................. 2
1.3 CDI 操作模式.................................................................................. 4
1.3.1 电化学实验与双电极电池对海水淡化的比较...................... 4
1.3.2 基于双电极布局的 CDI 测试几何模型..................................5
1.3.3 单通实验与批处理模式实验...................................................6
1.3.4 基于电极间电荷转移的建模...................................................8
1.3.5 概述碳/电解质界面的电化学反应和过程............................. 9
1.4 本论文的研究内容........................................................................10
第 2 章 实验方法................................................................................... 12
2.1 原料和试剂.................................................................................... 12
2.2 实验仪器、型号以及测试方法....................................................12
2.2.1 测试方法................................................................................. 13
第 3 章 椰壳炭用于电容去离子技术的研究...................................... 17
3.1 前言................................................................................................ 17
3.2 实验部分........................................................................................ 18
3.2.1 椰壳炭的制备......................................................................... 18
3.2.2 电极的制备............................................................................. 18
3.2.3 CDI 模块组装......................................................................... 18
3.2.4 装置的组装............................................................................. 18
3.3 结果与讨论.................................................................................... 19
3.3.1 不同水流速对 CDI 效果的影响............................................19
3.3.2 不同施加电压对 CDI 效果的影响........................................20
3.3.3 椰壳炭电极在不同盐溶液中的吸附性能.............................21
3.4 本章小结........................................................................................ 21
第 4 章 混合电容去离子技术的研究...................................................23
4.1 前言................................................................................................ 23
4.2 实验部分........................................................................................ 23
4.2.1 Na4Mn9O18的制备.................................................................. 23
4.2.2 电极的制备............................................................................. 24
4.2.3 电极的电化学表征................................................................. 24
4.2.4 脱盐性能测试......................................................................... 24
4.3 结果与讨论.................................................................................... 25
4.3.1 NMO 和 AC 电极的循环伏安测试.......................................25
4.3.2 HCDI 系统的脱盐性能测试.................................................. 26
4.3.3 不同操作模式下 HCDI 系统的性能.....................................27
4.3.4 HCDI 系统的充放电测试...................................................... 28
4.4 本章小结........................................................................................ 30
第 5 章 结论与展望............................................................................... 31
5.1 结论................................................................................................ 31
5.2 展望................................................................................................ 31
参考文献................................................................................................... 32
作者简介................................................................................................... 39
致 谢........................................................................................................40
第1章绪论
1.1前言
洁净水的供应是21世纪的关键技术、社会和经济挑战之一[1].联合国承认洁净水是一项基本人权,但全世界每七个人中就有一个人得不到洁净水.更复杂的是,全球范围内地下水开采的增加导致井水和含水层的咸水侵入.因此,人们对开发具有经济吸引力的海水淡化技术非常感兴趣.目前除了蒸馏、反渗透和电渗析技术外,需研究一个使这些技术在海水去离子方面更节能、更经济.考虑到世界上的咸水比淡水多,利用大量的咸水资源供人类消费和居住、农业和工业是特别有吸引力的.
电容去离子化(CDI)是近年来发展起来的一种更节能、更经济的低盐或中盐海水淡化技术.对于盐浓度低于10g/L的水,CDI能够将盐离子(水中的少数化合物)从混合物中除去.相反,其他方法从盐溶液中提取大多数相,即水.此外,在电极再生过程中的能量释放(离子释放或电极放电)可以被用来给在离子电吸附过程进行充电,从而实现能量回收.正如我们稍后将详细解释的,CDI循环由两个步骤组成,第一个步骤是离子电吸附(或充电)净化水的步骤,其中离子被固定在多孔碳电极对中.在接下来的步骤中,离子被释放,也就是说,从电极上被解吸,从而电极被再生.
在以前多孔碳电极的研究主要集中于电容储能器件,电容去离子化在一定程度上被忽略了.尽管多孔碳电极在海水淡化中的应用较早,自1960年代它被称为"电化学除盐作用"或"electrosorb过程脱盐水"[2-6],直到最近对这项技术的学术兴趣呈指数增加,一些公司已经开始营销商业CDI技术.
CDI使用一对相对放置的多孔碳电极,它们会在施加电压差后存储离子,见图1.1.这样的电极就可以一对一对地组装成多对.离子从流经两个电极之间的"间隔通道"的水中获取,并且固定在碳材料内部的孔隙当中.在这一过程的基础之上,形成了双电层(EDL).而EDL的形成是电容储能的基础,也是盐离子固定和选择性提取的机理.吸附一段时间后,所有孔隙体积都被电吸附离子饱和,达到设备的存储容量.为了使碳电极再生,离子通过降低甚至逆转 电池电压从电极中释放出来.这样,一股富含离子的小电流就产生了,离子吸 收能力被电极恢复了.
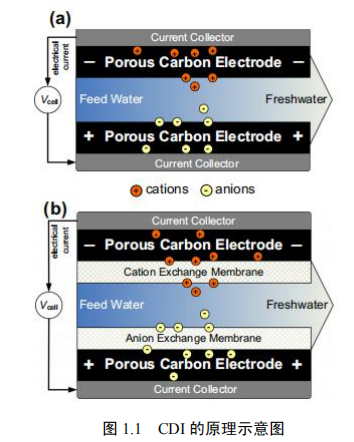
1.2CDI的历史背景
关于海水淡化概念的开创性工作,在当时被称为水的电化学脱盐,由Blair和Murphy[7-9]及同事在20世纪60年代初完成,并一直持续到20世纪60年代末.在那段时期,它是假定离子只能从水中移除特定化学组在场的时候表面上可以进行还原或氧化,其次是建立在溶液中离子之间的离子键和碳表面电离小组.根据Blair等人[10]的一项研究,大多数石墨类材料和其他形式的碳,当用作电极材料是由于存在对苯二酚偶联和其他被认为具有阳离子选择性的表面基团,对阳离子反应灵敏.
几年后,埃文斯和汉密尔顿用库仑-量测法及质量平衡分析法研究了电化学脱盐的机理.此研究提到在没有外部电压差的情况下离子吸附的程度.Evans等人也解释了CDI去除离子的机理.埃文斯认为在水的脱盐矿反应的第一步法拉第反应需要在阴极侧,以吸附氢和产生羟基离子.下一步,羟基离子所创造的基本条件为弱酸基团的电离提供了适当条件,从而根据离子交换的机制进行脱盐反应.在这样的再生过程当中,为了降低局部pH值,电压被反转,从而释放出之前被除去的离子.基于这一机理,要获得一个功能的脱盐循环,就必须在再生循环中逆转电压差,同时认为脱盐的效率取决于表面基团的浓度.解释多孔电极海水淡化原理的理论经过不断的发展,CDI的原理现可以简要总结如下,即离子是存储在电极的双电层电容中,所以碳基电极适合作为阳极和阴极,CDI过程的首要因素不再是法拉第反应,而是双电层电容[11-13].
对基于电容机理的脱盐过程Murphy和Caudle首次进行了详细的数学描述.将质量平衡方程与传质方程相结合,将盐浓度描述为时间的函数,并利用该模型描述了几种情况下的实验数据.Murphy等人[14]的另一项研究关注的是化学处理后用作电极材料的碳材料的表面特性,并且描述了对工艺效率的影响.Murphy等人得出结论,由于羧基在酸性处理后加入到表面,阳离子反应电极表现为离子交换器.Evans,etal,Accomazzo对电化学脱盐的机理和转运过程进行了较为详细的研究[15,16].
1968年,Reid等人的一项研究表明,CDI脱盐装置具有商业价值和长期使用价值.结果表明,除钠离子、氯离子以外,钙离子、镁离子、硫酸盐离子、硝酸盐离子及磷酸盐离子可被有效去除[17,18].
突破研究电化学除盐作用的概念是1970年代初由Johnsonetal[19]提出的理论被称为双电层理论(EDL),对离子去除的实际机制及使用操作条件做了介绍.它表明,通过使用不等的半周期时间或优化电池电压淡化步骤,可以提高系统性能,而不逆转极性.在同一项研究中,作者强调,固体导电材料与溶液界面可能发生的法拉第反应可能导致电极退化,从性能效率的角度来看,当电流主要是电容性流动时,这些过程不是必需的.此外,一项简单的成本研究首次表明,只要能生产出稳定的电极,CDI就具有经济可行性.对于多孔电极模型的发展Johnson等人做了的进一步研究,表明该模型用于分析离子,在多孔碳中的吸附及其电荷电压依赖性.结论是,电极的离子容量取决于双层膜的电容量、有效表面积和施加的电池电压[20].
1.3CDI操作模式
1.3.1电化学实验与双电极电池对海水淡化的比较
对于CDI电极的表征和测试,文献中描述了两种不同的方法.第一种方法是进行电化学(EC)分析,通常基于工作电极、计数器和参考电极组成的装置.这种方法只测量电流和电压信号,不测量盐浓度的实际变化,含盐离子的水不一定沿多孔碳电极(工作电极)流动或穿过多孔碳电极.这三个电极通常是不同的,只有工作电极由CDI材料制成,有待测试[21-23].
在另一种完全不同的方法中,含有盐的水沿着或通过由两个多孔碳电极组成的电池对流动,这两个多孔碳电极通常由相同的材料制成,在质量和尺寸上相等,在这两个多孔碳电极之间施加一个电池电压.在这个装置中可以测量海水淡化的程度以及对一定电池电压的电流响应.
第一种方法的使用源于超级电容器应用中类似的单电极测试,以及法拉第反应发生的(平面)电极研究.在一个三电极实验中,被研究电极的电压相对于参考电极被测量,作为工作电极和参比电极之间电流的函数.CDI电极测试中最常用的是:(1)循环伏安法(CV),扫描(工作)电极电位在较低和较高的点之间的电压,以及由此产生的电流绘制在电压电流曲线;(2)电化学阻抗谱(EIS),一个小电压信号,呈现正弦变化随着时间的推移,不同窗口频率有不同响应电流(也呈现正弦变化,但时间间隔),用于分析电极的尼奎斯特图,获得电阻的大小.
虽然这些技术已经被很好地建立了,但它们在多孔电极上的应用要比在平面电极上复杂得多,分析需要理解分布式电荷/渗透网络[24,25].从这些方法得到的数据的经典分析假设电极中的局部电阻不随时间和位置的变化而变化.然而,在以脱盐为主要目的的CDI中,这一条件并不满足,导致电极中强盐浓度随时间和地点而变化.
使用施加于两个电极之间的电池电压作为主要电信号的对称双孔电极电池进行测试.我们注意到,第二种方法也更容易让人想起使用CDI进行实际操作和脱盐,这是使用这种方法的另一个原因.
…………由于本文篇幅较长,部分内容省略,详细全文见文末附件





第5章结论与展望
5.1结论
1.椰壳炭电极在不同盐溶液流速下,溶液电导率首先下降较快,接着逐渐趋于平缓.电导率随着溶液流速的增加下降较快,但是继续增加流速至35r时,CDI效果变差.30r(10mL/min)的流速为最优.
2.电极在测试电压为1V时吸附效果最好,电导率最低降至950μS/cm.电极对高电荷、半径小的离子吸附量大.
3.NMO/AC非对称系统最大除盐率可达到0.066mg(NaCl)/g/s.
4.Na4Mn9O18具有较大的充电容量(40mAh/g~50mAh/g),NMO/AC非对称系统300圈循环后库仑效率接近100%,电化学性能稳定.
5.2展望
综上所述,我们认为CDI是一个具有挑战性和令人兴奋的领域,即使经过50年的发展,仍然可以被认为是一个新兴的技术.在了解CDI工艺方面仍然存在许多挑战,继续寻找新型电极材料作为提升CDI系统性能的解决方案.
参考文献
[1] 王瑗, 盛连喜, 李科, 等. 中国水资源现状分析与可持续发展对策研究[J]. 水 资源与水工程学报,2008, 50(3): 10-14.
[2] Abdel-Aal E A, Farid M E, Hassan F S M, et al. Desalination of Red Sea water using both electrodialysis and reverse osmosis as complementary methods[J]. Egyptian Journal of Petroleum, 2015, 24(1): 71-75.
[3] Johnson A M, Venolia A W, Wilbourne R G, et al. The electrosorb process for desaltingwater[J]. Washington: U.S. Dept. of the Interior, 1970, 17(3): 56-58.
[4] Johnson A M, Newman J. Desalting by means of porous carbon electrodes[J]. Electrochem Soc, 1971, 118(5): 7-10.
[5] Liu Y S, Chuan-Jun W U. Situation of land-water resources and analysis of sustainable food security in China[J]. Journal of Natural Resources, 2002, 19(3): 154-156.
[6] Opricovic S. A compromise solution in water resources planning[J]. Water Resources Management, 2009, 23(8): 1549-1561.
[7] Blair J W. Murphy G W. Mathematical theory of electrochemical demineraliza- tion in flowing systems[J]. Electrochim Acta, 1967, 12(16): 55-64.
[8] Murphy GW, Caudle DD. Mathematical theory of electrochemical demineralization in flowing systems[J]. Electrochim Acta, 1967, 12(16): 55-64.
[9] 王浩歌, 王小娟. 电渗析海水淡化技术研究进展[J]. 广东化工, 2017, 44(20): 138-140.
[10] Blair JW, Murphy GW. Electrochemical demineralization of water with porous electrodes of large surface area[J]. Saline water conversion. American Chemical Society, 1960, 20(6): 23.
[11] Ophir A. Low temperature, multi-effect distillation for cogeneration yielding the most efficient sea water desalination system[J]. Desalination, 1991, 84(s1-3): 85-101.
[12] Murphy GW, Cooper JL, Hunter JA. Activated carbon used as electrodes in electrochemical demineralization of saline water[J]. Washington: U.S. Dept. of the Interior, 1969, 35(17): 125-130.
[13] Eziyi I, Krothapalli A, Osorio J D, et al. Effects of salinity and feed temperature on permeate flux of an air gap membrane distillation unit for sea water desalination[C]. Technologies for Sustainability. IEEE, 2013, 20(7): 142-145.
[14] Kawada I. Development of High Efficiency Sea Water Desalination RO Membrane[J]. Membrane, 1999, 24(6): 336-341.
[15] Evans S, Hamilton W S. The mechanism of demineralization at carbon electrodes[J]. J Electrochem Soc, 1966, 11(3): 1314-1319.
[16] Anderson M A, Cudero A L, Palma J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete[J]. Electrochimica Acta, 2010, 55(12): 3845-3856.
[17] Reid G W, Townsend F M, Stevens A M. Filed operation of a 20 gallons per day pilot plant unit for electrochemical desalination of brackish water[J]. Washington: U.S. Dept. of the Interior, 1968, 8(6): 56-58.
[18] Hosseini S M, Madaeni S S. The Role of Nanomaterials in Water Desalination[J]. Nanocomposite Electrodialysis Ion-Exchange Membranes, 2017, 56(4): 76.
[19] Johnson AM, Newman J. Desalting by means of porous carbon electrodes[J]. Electrochem Soc, 1971, 11(8): 510-517.
[20] Ioannis C. Karagiannis, Petros G. Soldatos. Water desalination cost literature: review and assessment[J]. Desalination, 2008, 5(7): 77-81.
[21] 王宏涛, 李保安, 刘兵. 海水淡化技术现状及新技术评述[J]. 盐业与化工, 2014, 43(6): 1-5.
[22] 李长海, 张雅潇. 海水淡化技术及其应用[J]. 电力科技与环保, 2011, 27(1): 48-51.
[23] Sadrzadeh M, Mohammadi T. Sea water desalination using electrodialysis[J]. Desalination, 2008, 221(1): 440-447.
[24] Manfred L. System for desalination of sea water by electromagnetic sepn, DE19609384[P]. 1996, 33(5): 15-19.
[25] Hong L, Gang W, Wang L, et al. Capacitive deionization (CDI) technology for desalination of sea water:properties of carbon electrode materials made of activated carbon and ion-exchange membranes[J]. Ciesc Journal, 2012, 63(5): 67-68.
[26] Oren Y. Capacitive deionization (CDI) for desalination and water treatment-past, present and future (a review)[J]. Desalination, 2008, 228(1): 10-29.
[27] Yoon S W. Removal of Inorganic Ions from Ground Water by Capacitive Deionization[J]. 2008, 213(7): 10-13.
[28] Yoshinobu Tanaka, Reo Ehara, Sigeru Itoi, et al. Ion-exchange membrane electrodialytic salt production using brine discharged from a reverse osmosis seawater desalination plant[J]. Journal of Membrane Science , 2003, 6(1): 55-57.
[29] Zhang C, He D, Ma J, et al. Faradaic reactions in capacitive deionization (CDI) - problems and possibilities: A review[J]. Water Research, 2017, 12(8): 314.
[30] Toraj Mohammadi, Anita Kaviani. Water shortage and seawater desalination by electrodialysis[J]. Desalination, 2003, 7(1): 46-49.
[31] Nagel H W. DISTILLATION DESALINATION OF SEA WATER USING HEATED METAL BALLS TO VAPORIZE WATER: US, US3459638[P]. 1969, 76(4): 131-138.
[32] .Thamilselvan A, Nesaraj A S, Noel M. Review on carbon-based electrode materials for application in capacitive deionization process[J]. International Journal of Environmental Science & Technology, 2016, 13(12): 2961-2976.
[33] 高从堦, 周勇, 刘立芬. 反渗透海水淡化技术现状和展望[J]. 海洋技术学报, 2016, 35(1): 1-14.
[34] H?rtel A, Janssen M, Samin S, et al. Fundamental measure theory for the electric double layer: implications for blue-energy harvesting and water desalination[J]. Journal of Physics Condensed Matter An Institute of Physics Journal, 2015, 27(19): 129-139.
[35] Ma J, Du M. Multistage Flashing (MSF) System for Sea Water Desalination and Development of Domestic Device[J]. Hebei Electric Power, 1998, 55(12): 5-7.
[36] Liu D, Huang K, Xie L, et al. Relation between operating parameters and desalination performance of capacitive deionization with activated carbon electrodes[J]. Environmental Science Water Research & Technology, 2015, 1(4): 516-522.
[37] Kim T, Dykstra J E, Porada S, et al. Enhanced charge efficiency and reduced energy use in capacitive deionization by increasing the discharge voltage[J]. Journal of Colloid & Interface Science, 2015, 4(46): 317-326.
[38] Porada S, Bryjak M, Wal A V D, et al. Effect of electrode thickness variation on operation of capacitive deionization[J]. Electrochimica Acta, 2014, 2(24): 9313-9321.
[39] Taeyoung Kim, Hyun Deog Yoo, et al. Potential Sweep Method to Evaluate Rate Capabilityin Capacitive[J]. Electrochimica Acta, 2014, 3(39): 374-380.
[40] Suss M E. Size-based ion selectivity of micropore electric double layers in capacitive deionization electrodes[J]. Journal of the Electrochemical Society, 2017, 164(9): 73-77.
[41] Wen X, Zhang D, Shi L, et al. Three-dimensional hierarchical porous carbon with a bimodal pore arrangement for capacitive deionization[J]. Journal of Materials Chemistry, 2012, 22(45): 35-44.
[42] Landon J, Gao X, Kulengowski B, et al. Impact of Pore Size Characteristics on the Electrosorption Capacity of Carbon Xerogel Electrodes for Capacitive Deionization[J]. Journal of the Electrochemical Society, 2012, 159(11): A1861- A1866.
[43] Gaikwad M S, Balomajumder, Chandrajit. Polymer coated Capacitive Deionization Electrodefor Desalination: A mini review[J]. Electrochemical Energy Technology, 2016, 2(1): 1-5.
[44] Wang Y, El-Deen A G, Li P, et al. High-Performance Capacitive Deionization Disinfection of Water with Graphene Oxide-graft-Quaternized Chitosan Nanohybrid Electrode Coating[J]. Acs Nano, 2015, 9(10): 10-12.
[45] Jae-Hwan Choi. Fabrication of a carbon electrode using activated carbon powder and application to the capacitive deionization process[J]. Separation and Purification Technology, 2010, 70(7): 362-366.
[46] Yasin A S, Mohamed I M A, Mousa H M, et al. Facile synthesis of TiO2/ZrO2 nanofibers/nitrogen co-doped activated carbon to enhance the desalination and bacterial inactivation via capacitive deionization[J]. Scientific Reports, 2018, 8(14): 505-512.
[47] Farmer J C, Richardson J H, Fix D V, et al. Desalination with carbon aerogel electrodes. Revision[J]. Desalination with Carbon Aerogel Electrodes, 1996, 6(8): 55-58.
[48] Xu P, Drewes J E, Heil D, et al. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology[J]. Water Research, 2008, 42(10): 2605-2617.
[49] Richardson J H, Farmer J C, Fix D V, et al. Desalting in wastewater reclamation using capacitive deionization with carbon aerogel electrodes[J]. Office of Scientific & Technical Information Technical Reports, 1996, 15(4): 595-599.
[50] Quan X, Fu Z, Yuan L, et al. Capacitive deionization of NaCl solutions with ambient pressure dried carbon aerogel microsphere electrodes[J]. Rsc Advances, 2017, 7(57): 75-82.
[51] Zhang D, Wen X, Shi L, et al. Enhanced capacitive deionization of graphene/mesoporous carbon composites[J]. Nanoscale, 2012, 4(17): 544.
[52] Peng Z, Zhang D, Shi L, et al. High performance ordered mesoporous carbon/carbon nanotube composite electrodes for capacitive deionization[J]. Journal of Materials Chemistry, 2012, 22(14): 6603-6612.
[53] Wang H, Shi L, Yan T, et al. Design of graphene-coated hollow mesoporous carbon spheres as high performance electrodes for capacitive deionization[J]. Journal of Materials Chemistry A, 2014, 2(13): 4739-4750.
[54] Tsouris C, Mayes R, Kiggans J, et al. Mesoporous Carbon for CapacitiveDeionization of Saline Water[J]. Environmental Science & Technology, 2011, 45(23): 43.
[55] G Wang, B Qian, Q Dong, et al. Highly mesoporous activated carbon electrode for capacitive deionization.Separation & Purification Technology, 2013, 103(103): 216-221.
[56] Ma J, Wang L, Yu F. Water-enhanced performance in capacitive deionization for desalination based on graphene gel as electrode material[J]. Electrochimica Acta, 2018, 33(23): 30-35.
[57] Chung S, Kang H, Ocon J D, et al. Enhanced electrical and mass transfer characteristics of acid-treated carbon nanotubes for capacitive deionization[J]. Current Applied Physics, 2015, 15(11): 1539-1544.
[58] Li H, Pan L, Lu T, et al. A comparative study on electrosorptive behavior of carbon nanotubes and graphene for capacitive deionization[J]. Journal of Electroanalytical Chemistry, 2011, 653(2): 40-44.
[59] Zheng J, Xia L, Liu Y Y, et al. Review on the composite electrode of graphene for capacitive deionization technology[J]. Carbon Techniques, 2017, 633(7): 89-92.
[60] Li H, Zou L, Pan L, et al. Novel graphene-like electrodes for capacitive deionization[J]. Environmental Science & Technology, 2010, 44(22): 86-92.
[61] Chen M M, Wei D, Chu W, et al. One-pot synthesis of O-doped BN nanosheets as capacitive deionization electrode for efficient removal of heavy metal ions from water[J]. Journal of Materials Chemistry A, 2017, 5(32): 45-47.
[62] Wang G, Pan C, Wang L, et al. Activated carbon nanofiber webs made by electrospinning for capacitive deionization[J]. Electrochimica Acta, 2012, 69(5): 65-70.
[63] Limin Chang, Jiarun Lia, Xiaoyue Duan, et al. Porous carbon derived from Metal-organic framework (MOF) for capacitive deionization electrode[J]. Electacta Acta, 2015, 69(5): 73-76.
[64] Wang M, Huang Z-H, Wang L, et al. Electrospun ultrafine carbon fiber webs for electrochemical capacitive desalination[J]. New J Chem, 2010, 34(18): 43-45.





