摘要:骨细胞能够通过其机械转导功能协调骨骼对机械负荷的适应性反应,以维持骨稳态。随着年龄的增长,骨细胞及其微环境会出现一系列衰老相关的变化。衰老可能损伤骨细胞机械转导功能,引起下游信号的改变,造成骨重塑不平衡,进而导致骨质疏松、脆性骨折等退行性骨病的发生。笔者从“骨细胞复杂的机械转导过程”“骨细胞及其微环境的衰老相关改变”以及“衰老引起的骨细胞机械转导相关信号通路的改变”3个方面进行了综述,并对目前的研究难点和未来的研究方向进行总结和展望,以期为相关研究和衰老骨病防治提供新思路。
关键词:骨细胞;衰老;机械转导;骨重塑;骨质疏松;
Abstract:Osteocytes may coordinate the adaptive response of bone to mechanical loading through their roles in mechanotransduction to maintain bone homeostasis. Aging causes a series of changes in osteocytes and their microenvironment that may impair osteocyte mechanotransduction, leading to the imbalance of bone remodeling and the occurrence of degenerative bone diseases, such as osteoporosis and fragile fractures. In this review, we summarizes the mechanism of osteocyte mechanotransduction and discusses the aging-related changes of osteocytes and their microenvironment and the signaling pathways related to aging-related mechanotransduction impairment. Moreover, we summarizes the challenges in current research of osteocyte aging and forecasts future direction for the future investigation and the treatment of aging bone diseases.
Keyword:osteocyte; aging; mechanotransduction; bone remodeling; osteoporosis;
骨质疏松症(osteoporosis, OP)是一种与衰老密切相关的骨骼疾病,以骨量减少、骨微结构受损、骨强度下降及脆性骨折风险增加为主要特征[1]。在中国,约5.0%的男性和20.6%的女性在年龄大于40岁时患有OP,其常见并发症椎骨骨折的患病率为男性∶女性=10.5%∶9.7%[2]。在美国,每年约有150万例骨折是由OP引起的[3]。在全球范围内,骨质疏松性骨折的发病率和死亡率不断增加,造成约580万伤残调整寿命年数的损失[4]。由于OP所带来的社会经济负担日益突出[5],研究人员一直致力于探索有效的治疗方法[6],而深入了解衰老引起骨丢失背后的生物学机制对这一目标的实现尤为重要。
骨细胞来源于成骨细胞,是骨中最丰富、最长寿的细胞类型,占成人骨骼细胞总量的90%~95%[7]。骨细胞负责协调骨骼对机械负荷的适应性反应,通过分泌调节因子间接平衡破骨细胞介导的骨吸收和成骨细胞介导的骨形成,调节骨重塑、维持骨稳态[8,9]。随着年龄的增长,骨细胞的机械敏感性和机械转导功能下降,对局部成骨/破骨细胞的募集能力降低,由此引起的骨重塑失衡可能增加OP发病的风险[10]。考虑到更深入、更全面地理解衰老引起骨细胞机械转导功能退化的机制可能有助于找到防治老年OP或脆性骨折的有效靶点,笔者对相关研究进行综述。
1 骨细胞的机械转导功能
骨骼通过调整骨量以适应机械负荷的变化。适当增加机械负荷可使成骨细胞的成骨效应增强,骨量增加[11,12];反之,失去机械刺激则会抑制骨形成[13,14]、促进破骨细胞介导的骨吸收而造成骨量下降[15,16]。骨对机械负荷的适应性变化依赖于机械转导过程。
机械转导是指将组织水平的机械应变转化为局部生物物理信号以促进骨骼适应性反应的复杂生理过程,主要包括4个阶段:将组织水平的载荷转化为机械敏感细胞可感知的局部力学信号(机械耦合)、将机械敏感细胞中的力学信号转导为生化反应(生化偶联)、从机械感觉细胞到效应细胞的信号传递以及后续效应细胞的反应过程[17]。骨细胞是骨内主要的机械感觉细胞,可发挥机械转导作用,在局部微环境中协调后续的组织反应[9,18]。
骨细胞感知机械负荷的方式包括以下3种[19,20]。首先,骨细胞通过腔隙-小管网络(lacuna-canalicular network, LCN)相互连接[21]。机械负荷引起的骨基质变形会改变LCN内流体流动的速率,骨细胞感受到这种剪切应力变化后[22],可分泌信号因子调节成骨细胞和破骨细胞的活性,协调对机械负荷的适应性反应[22,23,24]。其次,机械负荷诱导的液体压力也可激活骨细胞。低至13千帕的间歇性流体静压即可诱导骨细胞持续释放前列腺素E2(prostaglandin E2,PGE2)[24]。另外,骨细胞还可能对机械负荷引起的骨基质应变(拉伸应变)作出直接反应[24,25]。
生化偶联过程包括以下4种可能的机制。第一种由骨细胞膜上的整合素介导。整合素可与黏着斑激酶结合,将力传递给细胞外因子,促进应力纤维形成[26]。机械负荷诱导的剪切应力变化可引起整合素的构象变化,激活其下游信号传导,改变蛋白质的活性和表达[16,27]。第二种由初级纤毛参与。初级纤毛从细胞表面突出,直接接触细胞外环境,在化学和机械感觉中起关键作用[28]。初级纤毛参与骨细胞机械信号与钙信号的偶联过程[29]。破坏其结构会降低细胞对流体流动的反应,使机械诱导的骨桥蛋白mRNA表达、细胞外PGE2水平以及骨保护素与核因子κB受体活化因子配体的mRNA比率降低[30]。第3种涉及膜通道。骨细胞对机械刺激最早的反应之一是细胞内钙离子(Ca2+)浓度升高,抑制骨细胞T型电压敏感的钙通道或钆敏感的拉伸激活通道显着减少了细胞质中Ca2+动力学反应的数量[31,32]。瞬时受体电位香草素受体4(transient receptor potential vanilloid 4,TRPV4)通道是流体流动诱导的剪切力增加细胞质Ca2+水平、减少硬化素(sclerostin, SOST)表达的关键组成部分[33]。第4种与缝隙连接有关。由缝隙连接蛋白43(connexin, Cx43)形成的缝隙连接可作为骨细胞响应机械负荷而产生信号的通道[34]。但也有研究不支持这一观点,认为骨细胞中Cx43基因(Gja1)特异性缺陷的小鼠对机械负荷更敏感[35,36]。然而,这种相悖结果背后的机制尚不清楚。
2 骨细胞及其微环境的衰老相关改变
衰老引起的骨重塑失衡可导致骨骼机械性能退化、骨量减少以及骨微结构的改变[37,38,39]。骨微结构改变主要表现为骨小梁数量减少、间距增加以及厚度减少[40,41]。衰老引起强烈的皮质内侧骨吸收和响应性的骨膜侧骨形成,但由于吸收率大于形成率,导致皮质变薄和骨髓腔扩张[1]。此外,皮质骨孔隙率也随衰老而显着增加,骨脆性增强,在绝经后女性中更为明显[39,42]。与此同时,骨细胞及其微环境也发生变化,影响骨细胞的机械转导功能。
2.1 骨细胞的变化
衰老会破坏骨细胞网络的形态和完整性。衰老小鼠(18个月龄)的骨细胞较年轻小鼠(3个月龄)出现了胞质溶解和细胞核异常等形态改变[8]。相比年轻小鼠骨细胞丰富的树突网络,衰老小鼠的骨细胞仅有少量连续的树突[8]。与5个月龄小鼠相比,22个月龄小鼠的股骨骨细胞密度下降了20%~30%,树突也显着减少,且树突减少先于骨细胞减少,提示树突丢失可能降低了骨细胞的生存能力[43]。对20~73岁健康女性的髂嵴活检结果显示骨细胞及其腔隙密度随年龄增长而降低,且腔隙数量始终高于骨细胞数量,说明衰老骨中存在空腔隙[44]。
2.2 LCN的变化
骨细胞的树突相互连接形成网络,并外延至脉管系统和皮质内层,其结构基础为LCN[45]。LCN具有重要生理意义。小管内流体可通过LCN将营养物质及各种物理、化学和生物信号因子传递给骨细胞,调节骨细胞功能[46]。因此,LCN是骨组织正常力学响应的基础。年轻时细长、定向排列的骨细胞腔隙在衰老状态下变得圆而无序[43,47]。不论男性或女性,股骨骨细胞的腔隙密度均随年龄增长而减少[48]。老年人骨细胞的腔隙密度较年轻人显着降低15%~30%[49,50]。此外,衰老骨细胞的树突和小管数量也显着减少[43,51],进一步损害了LCN的连通性。衰老引起的LCN改变可能影响骨细胞的机械转导功能。
2.3 骨基质的变化
骨基质主要由矿物质、胶原蛋白、水和少量的非胶原蛋白组成[52],是骨细胞正常机械转导的微环境基础。骨矿物质由结晶不良的碳酸磷灰石组成,其平均大小取决于骨组织的年龄。随着骨骼的老化,矿物晶体变得越来越长,结晶也越来越多[53]。结晶度增加使骨的极限应变和韧性降低,骨组织机械性能下降[53,54]。骨基质中的胶原蛋白含量、纤维之间和纤维内部的交联程度或性质也随衰老发生改变。骨基质Masson染色发现衰老骨基质的胶原组成发生变化[8]。天狼星红染色显示衰老骨基质中的胶原纤维组织结构疏松[8]。水占皮质骨体积的10%~20%[55],随衰老流失至5%[52]。细胞外结合水不仅能够滋养细胞,还可通过增加流动电位或细胞壁上的剪切力将机械信号从一个细胞传递到另一个细胞[52]。因此,水量流失会降低骨细胞的机械敏感性,妨碍机械转导。非胶原蛋白如糖胺聚糖,能够将胶原纤维结合在一起以稳定骨中胶原纤维[56],其含量随衰老而减少[57,58]。骨基质的衰老改变可能是由骨细胞衰老引起的,又或是独立于骨细胞功能变化的事件,也可能是骨细胞-骨基质交互作用的结果。而有关骨基质的衰老改变是如何影响骨细胞机械力学转导的问题,尚未深入研究。
3 衰老引起的骨细胞机械转导相关信号通路的改变
骨细胞机械转导是将感受到的机械应变信号转换为生物信号并激活相关信号通路的过程(见图1)。Wnt信号通路的激活是其中最重要的级联反应之一[21]。典型的Wnt信号通路由β-catenin介导[59]。β-catenin对成骨细胞分化、增殖和基质产生至关重要[60]。同时,它也是骨细胞的一个关键调节因子,与其机械反应性相关[61]。一项研究发现Wnt信号通路成分在衰老骨中显着下调,提示Wnt信号通路受损与机械转导功能降低之间可能存在联系[62]。SOST是一种来源于骨细胞的Wnt通路抑制因子,其过表达导致成骨细胞的功能抑制,骨形成减少[63]。该基因具有力学响应性,是骨细胞机械转导下游的效应分子。机械刺激可降低SOST的表达。衰老导致SOST表达增高,可能是衰老骨组织力学响应性受损的重要机制[64]。另一项研究提示骨细胞中三组与机械转导相关的基因的表达发生变化[8],包括感受机械负荷的关键分子,如整合素及其相关因子(整合素α10、整合素β4、Chad、成纤维细胞激活蛋白)、局部粘附剂成分(层粘连蛋白β2、血小板反应蛋白2/4、细胞粘合素C、细胞粘合素N、血小板衍生生长因子受体α)、细胞骨架蛋白(辅肌动蛋白α2、窖蛋白1、细丝蛋白C、肌球蛋白轻链2、肌球蛋白轻链激酶4)、构成骨细胞之间间隙连接的主要钙连接蛋白(Cx43/间隙连接蛋白α1);机械转导中的第二信使,包括参与一氧化氮、PGE2和三磷酸腺苷合成的关键酶(如神经元型一氧化氮合酶、环氧化酶1-3、烟酰胺腺嘌呤脱氧核苷酸脱氢酶第1-5亚单位)、钙稳态相关基因(如肌集钙蛋白1、Junctophilin2基因、triadin基因、连接性肌浆网蛋白1、组氨酸富集钙蛋白、钙蛋白酶激酶3、兰尼碱受体1);效应通路,如环磷酸鸟苷酸-环磷酸鸟苷酸依赖的蛋白激酶、磷脂酰肌醇3-激酶-蛋白激酶B和Wnt信号通路[8]。最近的研究发现,衰老小鼠(22个月龄)骨细胞中的钙离子信号较年轻小鼠(5个月龄)减弱,且在胫骨负荷期间,对钙离子信号的响应性减弱[65]。压电1型机械敏感性离子通道(Piezo1)是一种在机械转导中具有重要作用的钙通道,已被证实是调节骨骼稳态的主要骨骼机械传感器[66,67]。Piezo1可能参与了衰老相关的骨细胞机械转导改变。但这只是笔者的推测,尚无确切证据表明Piezo1在骨细胞衰老中的作用。
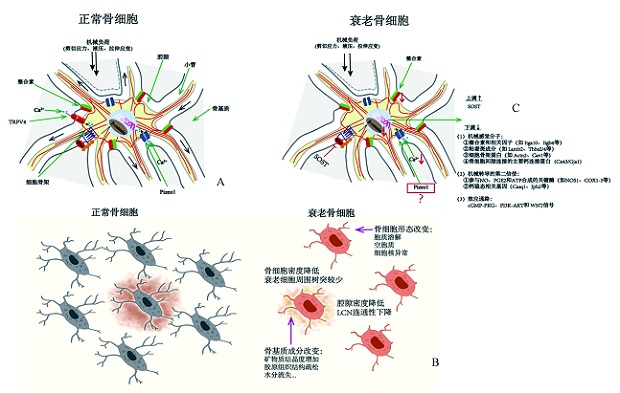
图1 衰老对骨细胞机械转导功能的影响
Fig.1 The effect of aging on osteocyte mechanotransduction A: Osteocyte mechanotransduction; B: Aging-related changes of osteocytes and their microenvironment; C: Effect of aging on gene expression related to osteocyte mechanotransduction.
A:骨细胞正常的机械转导过程;B:骨细胞及其微环境的衰老相关变化;C:衰老对骨细胞机械转导相关基因表达的影响。 注:图1来源于参考文献[68],TRPV4=瞬时受体电位香草素受体4通道,Pizeo1=压电1型机械敏感性离子通道,SOST=硬化素,Itga10=整合素α10,Itgb4=整合素β4,Lamb2=层黏连蛋白β2,Thbs2/4=血小板反应蛋白2/4,Actn2=辅肌动蛋白α2,Cav1=窖蛋白1,Cx43=缝隙连接蛋白43,Gja1=间隙连接蛋白α1,NOS1=神经元型一氧化氮合酶,COX1-3=环氧化酶1-3,Casq1=肌集钙蛋白1,Jph2=Junctophilin2基因,cGMP-PKG=环磷酸鸟苷酸-环磷酸鸟苷酸依赖的蛋白激酶,PI3K-AKT=磷脂酰肌醇3-激酶-蛋白激酶B。
4 总结与展望
骨细胞正常的机械转导功能对骨重塑的调节和骨稳态的维持至关重要。大量动物和人体研究已提供了衰老骨组织中骨细胞及其微环境改变的确实证据。由于这些结构、成分不同程度地参与了骨细胞的机械转导过程,其衰老相关改变不可避免会影响正常的机械转导,引起骨重塑失衡,并逐渐发展为OP。因此,衰老导致骨细胞机械转导功能改变的机制探索是揭示骨衰老和治疗骨衰老的重要环节。靶向重要的分子可以预防骨细胞机械转导功能下降导致的骨重塑不平衡,是一条合理的治疗思路。但现阶段有关这方面的分子机制研究仍然较少。一方面,从骨中分离骨细胞的技术难度很大,运用高通量基因检测技术探索衰老骨细胞相关基因表达及信号通路变化的研究仍然很少;另一方面,采用骨切片原位免疫组织化学或免疫荧光染色,虽然能够检测到某些基因表达的改变,但由于该技术受到骨组织本身特点及蛋白抗体质量的制约,适用的基因有限,某些情况下并不令人信服。利用体外骨细胞模型探索衰老骨细胞的机械生物学改变也存在一定的技术限制。衰老的体内环境及骨骼所受机械刺激的方向、大小和形式较为复杂,在体外难以完全模拟,这使得体外研究困难重重。因此,当下迫切需要从衰老骨中获取骨细胞的有效实验方案。现有的主流方案是通过单种或多种胶原酶消化联合乙二胺四乙酸脱钙,该方法虽已被证实可一定程度地分离部分骨细胞,但存在很多明显缺点,如骨细胞释放率低、崩解的骨基质残渣难以清除、消化时间长、消化所用的试剂对骨细胞的活性以及基因表达存在一定影响等,严重限制了该方法在骨细胞转录组研究中的应用。尽管如此,针对性地完善方案,如减少消化时间、增加消化频率、在消化溶液中加入保护细胞的因子及流式分选高活性细胞等,可相对弥补上述缺点。事实上,Wein等[69]已成功运用改进后的方案(减少消化时长、增加消化频率)获取到了部分骨细胞,尽管数目较少。还需注意的是,由于不同解剖位置的骨细胞所接受的机械刺激不同,所以皮质骨浅层和深层的骨细胞基因表达也可能存在明显差异。但毫无疑问,经过不断完善后的骨细胞分离方案必然会推动新兴的单细胞测序技术在骨细胞机械转导功能研究中的应用,衰老导致骨细胞机械转导功能退化的分子机制也将被逐步揭示,为临床治疗老年OP及脆性骨折等疾病提供理论依据和新策略。
参考文献
[1] Hemmatinan H,Bakker AD, Klein-Nulend J.et al.Aging. osteocytes, and mechanotransduction[J]. Curr Osteoporos Rep,2017.15(5):401-411.
[2] Wang L,Yu W,Yin X,et al.Prevalence of osteoporosis and fracture in China:the China osteoporosis prevalence study[J].JAMA Netw Open,2021. .4(8)。e2121106.
[3] Black DM,Rosen CJ. Clinical practice Postmenopausal osteoporosis[J].N Engl J Med,2016,374(3):254-262.
[4] Johnell O,Kanis JA. An estimate of the worldwide prevalence and disbility associated with osteoporotic fractures[J].Osteoporos Int,2006,17(12):1726-1733.
[5] Veronese N,Maggi S Epidemiology and social costs of hip fracture[J].Injury,2018.49(8):1458-1460.
[6] Estell EG,Rosen CJ.Emerging insights into the comparative effectiveness of anabolic therapies for osteoporosis[J] Nat Rev Endocrinol,2021,17(1):31-46.
[7] Bonewald LF.The amazing osteocyte[J].J Bone Miner Res ,2011,26(2):229-238.
[8] Zhang C,Xu s,Zhang S,et al.Ageing characteristics of bone indicated by transcriptomic and exosomal proteomic analysis of cortical bonecells[J].J Orthop Surg Res ,2019,14(1):129.
[9] Schaffler MB,Cheung WY,Majeska R,et al.Osteocytes.master orchestrators of bone[J].Calcif Tissue Int,2014,94(1):5-24.
[10] Klein-Nulend J,van Oers RF,Bakker AD,et al.Bone cell mechanosensitivity,estrogen deficiency,and osteoporosis[J].J Biomech,2015,48(5):855-865.
[11] Robling AG,Niziolek PJ,Baldridge LA,et al.Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin[J]J Biol Chem,2008,283(9):5866-5875.
[12] Turner CH,Owan I.Alvey T,et al.Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine[J]. Bone,1998,22(5):463-469.
[13] Nakamura H,Aoki K,Masuda W,et al. Disruption of NF-kappaB1 prevents bone loss caused by mechanical unloading[J]J Bone Miner Res ,2013,28(6):1457-1467.
[14] Kondo H,Nifuji A, Takeda S,.et al.Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss viasympathetic nervous system[J]J Biol Chem,2005,280(34):30192 -30200.
[15] Xiong J,Onal M,Jilka RL,et al.Matrix-embedded cells control osteoclast formation[J.Nat Med,2011,17(10):1235-1241.
[16] Uda Y,Azab E,Sun N,et al.Osteocyte mechanobiology[J].Curr Osteoporos Rep,2017, 15(4):318-325.
[17] Duncan RL,Turner CH.Mechanotransduction and the functional response of bone to mechanical strain[J] Calcif Tissue Int, 1995,57(5):344-358.
[18] Ehrlich PJ,Lanyon LE Mechanical strain and bone cell function:a review[J].Osteoporos Int,2002, 13(9)688-700.
[19] Klein-Nulend J, Bakker AD, Bacabac RG,et al. Mechanosensation and transduction in osteocytes[J].Bone,2013. 54(2):182-190.
[20] Klein-Nulend J, Bacabac RG, Bakker AD.Mechanical loading and how it affects bone cells:the role of the osteocyte cytoskeleton in maintaining our skeleton[J] Eur Cell Mater,2012,24:278-291.
[21] Robling AG,Bonewald LF.The osteocyte:.New insights[J].Annu Rev Physiol,2020,82.485-506.
[22] Fritton SP,Weinbaum S Fluid and solute transport in bone:Flow induced mechanotransduction[J] Annu Rev Fluid Mech,2009 ,41:347-374.
[23] Price C ,Zhou X,Li W,et al.Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone:direct evidence for load-induced fluid flow[J].J Bone Miner Res. 2011,26(2):277-285.
[24] Klein-Nulend J,van der Plas A,Semeins CM.et al.Sensitivity of osteocytes to biomechanical stress in vitro[J].FASEB J,1995,.9(5):441-445.
[25] Robinson JA,Chatterjee-Kishore M,Yaworsky PJ,et al.Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone[J].J Biol Chem,2006,281(42):31720-31728.
[26] Hamamura K,Swarnkar G,Tanjung N,et al. RhoA-mediated signaling in mechanotransduction of osteoblasts[J].Connect Tissue Res,2012, 53(5):398-406.
[27] Hoffman BD,Grashoff C,Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction[J] Nature ,2011,475(7356):316-323.
[28] Hoey DA,Chen JC,Jacobs CR. The primary cilium as a novel extracellular sensor in bone[J]. Front Endocrinol (L ausanne),2012,3:75.
[29] Xiao Z,Zhang S ,Mahlios J,et alCila-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression[J].J Biol Chem,2006. ,281(41):30884-30895.
[30] Malone AM,Anderson CT,Tummala P,et al.Primary cilla mediate mechanosensing in bone cells by a calcium-independent mechanism[J].Proc Natl Acad Sci U S A,2007,104(33):13325-13330.
[31] Brown GN,Leong PL,Guo XE.T-type voltage-sensitive calcium channels mediate mechanically-induced intracellular calcium oscillations in osteocytes by regulating endoplasmic reticulum calcium dynamics[J] Bone,2016,88:56-63.
[32] Miyauchi A,Notoya K,Mikuni-Takagaki Y,et al.Parathyroid hormone- activated volume-sensitive calcium influx pathways in mechanically 1oaded osteocytes[J].J Biol Chem,2000,275(5):3335-3342.
[33] Lyons JS,Joca HC,Law RA,et al.Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes[J] Sci Signal,2017,10(506):eaan5748.
[34] Cheng B,Zhao S,Luo J,et al. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells[J].J Bone Miner Res.2001,16(2):249-259.
[35] Grimston SK, Brodt MD,Silva MJ,et al.Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1)[J]J Bone Miner Res,2008,23(6):879-886.
[36] Bivi N,Pacheco-Costa R,Brun LR,et al.Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice[J].J Orthop Res,2013,31(7):1075-1081.
[37] Lai X.Price C,Modla S,et al.The dependences of osteocyte network on bone compartment,age,and disease[J].Bone Res,2015,3:15009.
[38] Milovanovic P,Zimmermann EA,Riedel C ,et al.Multi-level characterization of human femoral cortices and their underlying osteocyte network reveal trends in quality of young,aged,osteoporotic and antiresorptive -treated bone[J] Biomaterials,2015,45:46-55.
[39] Seeman E Age-and menopause-related bone loss compromise cortical and trabecular microstructure[J].J Gerontol A Biol Sci Med Sci,2013,68(10):1218-1225.
[40] Majumdar S, Genant HK, Grampp S, et al.Correlation of trabecular bone structure with age ,bone mineral density,and osteoporotic status:in vivo studies in the distal radius using high resolution magnetic resonance imaging [J]J Bone Miner Res, 1997,12(1)111-118.
[41] Ding M,Hvid 1.Quantification of age-related changes in the structure model type and trabecular thickness of human tibial cancellous bone[J] .Bone ,2000,26(3):291-295.
[42] Bilezikian JP, Kurland ES,Rosen CJ.Male skeletal health and osteoporosis[J].Trends Endocrinol Metab, 1999, 10(6):244-250.
[43] Tiede-Lewis LM,Xie Y,Hulbert MA,et al.Degeneration of the osteocyte network in the C57BL/6 mouse model of aging[J].Aging (Albany NY),2017,9(10):2190-2208.
[44] Qiu S,Rao DS,Palnitkar S,et al.Relationships between osteocyte density and bone formation rate in human cancellous bone[J] Bone,2002,31(6):709-711.
[45] Yee CS, Schurman CA,White CR, et al.Investigating osteocytic perilacunar/ canalicular remodeling[J] Curr Osteoporos Rep,2019,17(4):157-168.
[46] Tiede-Lewis LM,Dallas SL Changes in the osteocyte lacunocanalicular network with aging[J]. Bone ,2019, 122:101-113.
[47] Heveran CM,Rauff A,King KB,et al.A new open-source tool for measuring 3D osteocyte lacunar geometries from confocal laser scanning microscopy reveals age-related changes to lacunar size and shape in cortical mouse bone[J]. Bone,2018,110:115-127.
[48] Busse B,Djonic D,Milovanovic P,et al.Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone[J] Aging Cell,2010,9(6):1065-1075.
[49] Qiu S,RsO DS,Palnitkar S,et al.Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone[J] .Bone ,2002,31(2):313-318.
[50] Vashishth D,Verborgt 0,Divine G,et al.Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age[J]. Bone, 2000,26(4):375-380.
[51] Milovanovic P,Zimmermann EA,Hahn M,et al. Osteocytic canalicular networks.morphological implications for altered mechanosensitivity[J]. ACS Nano,2013,7(9):7542-7551.
[52] Burr DB. Changes in bone matrix properties with aging[J]. Bone,2019,120:85-93.
[53] Akkus 0.Adar F,Schaffler MB .Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone[J]. Bone,2004,34(3):443-453.
[54] Zioupos P,Currey JD.Changes in the stiffness, strength, and toughness of human cortical bone with age[J].Bone, 1998 ,22(1):57-66.
[55] Robinson RA. An electron-microscopic study of the crystalline inorganic component of bone and its relationship to the organic matrix[J].J Bone Joint Surg Am,1952 ,34-A(2):389-435.
[56] Bertassoni LE Swain MV.The contribution of proteoglycans to the mechanical behavior of mineralized tissues[J].J Mech Behav BiomedMater,2014,38:91-104.
[57] Wang X,Xu H,Huang Y,et al. Coupling effect of water and proteoglycans on the in situ toughness of bone[J]J Bone Miner Res 2016,31(5)-:1026- 1029.
[58] Wang X,Hua R,Ahsan A,et al.Age-related deterioration of bone toughness is related to diminishing amount of matrix glycosaminoglycans (Gags)[J]. JBMR Plus ,2018,2(3):164-173.
[59] Maeda K,Kobayashi Y,Koide M,et al.The regulation of bone metabolism and disorders by Wnt signaling[J].Int J Mol Sci,2019,20(22):5525
[60] Dallas SL,Prideaux M,Bonewald LF.The osteocyte:an endocrine cell and more[J]. Endocr Rev,2013,34(5):658-690.
[61] Bonewald LF,Johnson ML .Osteocytes mechanosensing and Wnt signaling[J] Bone ,2008 42(4):606-615.
[62] Galea GL,Meakin LB,Harris MA,et al.Old age and the associated impairment of bones' adaptation to loading are associated with transcriptomic changes in cellular metabolism,cell-matrix interactions and the cell cycle[J]. Gene ,2017,599:36-52.
[63] Winkler DG, Sutherland MK,Geoghegan JC,et al. Osteocyte control of bone formation via sclerostin,a novel BMP antagonist[J] EMBO J,2003,22(23)-6267-6276.
[64] Tu x,Rhee Y,Condon KW,et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading[J]. Bone,2012,50(1):209-217.
[65] Morrell AE,Robinson ST,Silva MJ,et al.Mechanosensitive Ca(2+) signaling and coordination is diminished in osteocytes of aged mice during ex vivo tibial loading[J].Connect Tissue Res ,2020.61(3-4):389-398.
[66] Wang L,You X,Lotinun S ,et al.Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk[J].Nat Commun,2020,11(1):282.
[67] Zhou T,Gao B,Fan Y,et al.Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-catenin[J] Elife ,2020,9:e52779.
[68] Murshid SA. The role of osteocytes during experimental orthodontic tooth movement:A review[J] Arch Oral Biol,2017,73:25-33.
[69] Wang JS, Kamath T,Mazur CM,et al.Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin[J].Nat Commun,2021,12(1):6271.





