摘 要: 发展了一种无催化剂催化的烯烃碘氧双官能团化反应,以烯烃、N-碘代丁二酰亚胺和3-羟基-1,2,3-苯并三嗪-4(3H)-酮为起始原料,通过加成反应实现了烯烃的3-碘2-氧双官能团化的三组分反应。该反应具有无需催化剂、条件温和、产率良好、区域选择性好和操作简单等优点,从而为含碘氧类化合物的高效绿色合成提供了新方法。
关键词: 烯烃; N-碘代丁二酰亚胺; 3-羟基-1,2,3-苯并三嗪-4(3H)-酮; 双官能团化反应;
Abstract: The iodine/oxygen bifunctionalizations of alkenes under catalyst-free condition have been developed. The three-component reaction of alkenes, 1-iodopyrrolidine-2,5-dione(NIS) and 3-hydroxybenzo[d][1,2,3]triazin-4(3 H)-one for synthesis of 3-iodinated 2-oxygenated bifunctionalization of alkenes was fulfilled through addition reaction. This method has advantages such as catalyst-free, mild reaction conditions, high yields, high regioselectivity and simple operations, providing a novel, efficient and green route to synthesize iodine and oxygen atom containing compounds.
Keyword: alkene; 1-iodopyrrolidine-2,5-dione; 3-hydroxy-1,2,3-benzotriazin-4(3H)-one; bifunctionalization;
烯烃作为一种常见的有机化合物,能与多种试剂发生加成反应,因而受到有机化学家和化工工作者的高度关注。烯烃的双官能团化反应能够在烯烃的两端添加两个不同的官能团从而完成化合物种类的转变,通常同时具有原子经济性和步骤经济性等优点,所以格外受到化学合成工作者的青睐[1,2]。但是烯烃的双官能团化反应常常会因为需要贵金属催化剂催化[3]、反应条件苛刻[4]、区域选择性差[5]、对环境不友好[6]和产率不高[7]等因素的影响而降低了其应用价值。烯烃的卤氧化双官能团反应是一种合成天然产物的重要途径,文献对该类反应有诸多报道,常见的卤氧双官能团化有TICA/甲醇[8],MX/H2O[5,9],HX/O[10]22[10],Me3SI/PhI(OAc)[11]22[11],MI/EtOH[12],PhCONHBr/H2O[13]和NaI/H2O[14]22[14]等反应体系,体系中的试剂虽然大多廉价易得,但是实现此类烯烃的卤氧双官能团化反应存在大多需要催化剂[12]、反应温度苛刻[13]和某些卤化试剂制备过程繁琐等缺点[8],因此如何高效、绿色、便捷地实现烯烃的卤氧双官能团化反应仍具有一定的挑战性和研究价值。
在查阅各种文献的基础上,笔者通过大量实验,发展了一种以烯烃、NIS和3-羟基-1,2,3-苯并三嗪-4(3H)-酮为原料在室温下一步合成3-碘-2-氧烯烃加成产物的碘氧双官能团化新反应。该反应无需任何催化剂,且具有良好的区域选择性和操作便捷性。
1、 实验部分
1.1、 实验仪器及试剂
仪器:1H NMR和13C NMR用Bruker Advance Ⅲ 500 MHz型核磁共振仪(TMS作为内标,CDCl3作为溶剂);反应过程用薄层色谱跟踪和分析,除特殊注明需要用碘缸显色外,其他的均用型号为ZF-I的三用紫外分析仪鉴别;柱色谱为柱层析硅胶(青岛海洋化工)。所有反应均在封管中进行,采用磁力搅拌。
主要溶剂和试剂均为市售分析纯或者化学纯,如无特殊说明无需处理。
1.2、 实验方法
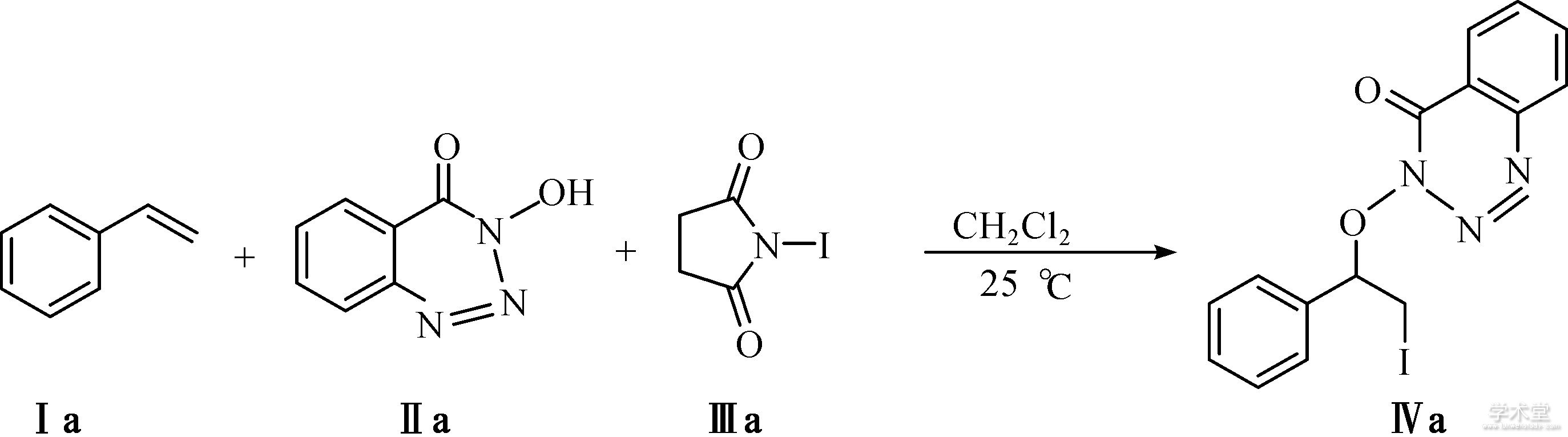
称取3-羟基-1,2,3-苯并三嗪-4(3H)-酮(0.5 mmol,0.082 g)、NIS(1.0 mmol, 0.225 g)和苯乙烯(1.0 mmol,0.104 g)依次加入到25 mL的封管中,用5 mL一次性进样针量取4 mL 1,2-二氯乙烷(DCE)作为溶剂,室温下(25 ℃)搅拌,24 h后点板显示反应结束。反应液用布氏漏斗过滤,用乙酸乙酯洗涤滤渣3 次(每次5 mL),滤液转移到50 mL的梨形瓶中,经旋蒸浓缩后用V(石油醚)∶V(乙酸乙酯)=15∶1柱层析分离得到浅黄色固体的目标产物3-(2-碘-1-苯基乙氧基)苯并[d][1,2,3]三嗪-4(3H)-酮,0.137 g,产率为70%。
2、 结果与讨论
2.1 、反应条件优化
以苯乙烯(1.0 mmol,0.104 g)、3-羟基-1,2,3-苯并三嗪-4(3H)-酮(0.5 mmol,0.082 g)和NIS(1.0 mmol,0.225 g)为模型反应的底物,以二氯甲烷(DCM)为溶剂,在室温(25 ℃)条件下反应24 h,成功分离得到了产率为50%的目标产物IVa,笔者在此基础上展开了进一步的反应条件优化。反应方程式为
2.1.1、 溶剂对反应的影响
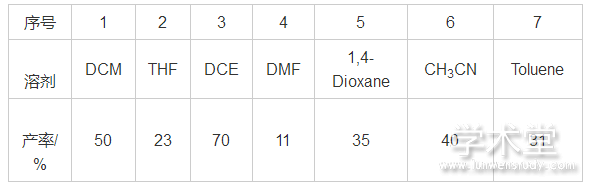
首先对反应的溶剂进行了筛选。在进而采用的THF,DCE,DMF,1,4-dioxane,CH3CN,Toluene等溶剂中,意外地发现与DCM具有类似结构的1,2-二氯乙烷(DCE)作为溶剂,能够获得较好的产率(70%),而反应在DMF中的产率最低(11%),结果如表1所示。
表1 溶剂对反应的影响
2.1.2、 温度对反应的影响
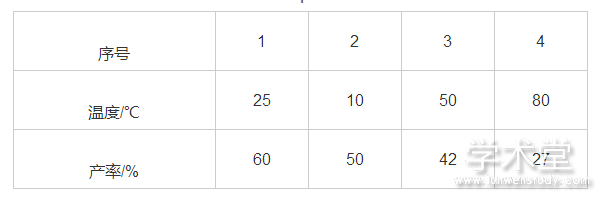
在以DCE为溶剂取得较优产率后,笔者尝试对反应温度进行筛选。实验结果表明降低温度对反应不利。这可能是因为温度降低减慢了反应的速率,同时反应温度升高导致产率降低或许与含碘化合物的热稳定性差有关,结果如表2所示。
表2 温度对反应的影响
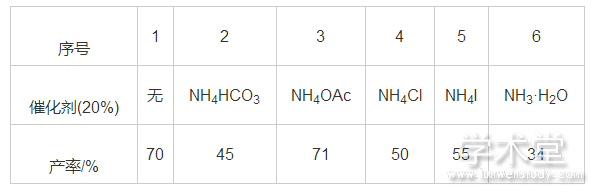
2.1.3、 催化剂对反应的影响
找到较优反应溶剂和反应温度后,笔者试图在反应催化剂上展开甄选以提高反应产率,在查阅文献基础上[15,16,17,18],选择了一些廉价的铵盐作为反应催化剂,如表3所示。实验结果表明:只有当采用摩尔分数为20%的醋酸铵为催化剂时,反应的产率(71%)与无催化剂反应体系的产率(70%)相当,但考虑到反应经济性,最终选择了不使用催化剂。
表3 催化剂对反应的影响
2.2、 底物拓展
在获得的最优反应条件下(苯乙烯(1.0 mmol,0.104 g),NIS(1.0 mmol,0.225 g),3-羟基-1,2,3-苯并三嗪-4(3H)-酮(0.5 mmol, 0.082 g,反应温度25 ℃),以DCE为溶剂,笔者对该烯烃的碘氧双官能团化反应的普适性进行了考察。结果发现:芳香烯烃芳环上无论连有吸电子基团还是供电子基团反应都能进行,产物具有较好的区域选择性;底物为间氯苯乙烯时,分离过程的Rf值为0.5,区域选择性较差,反应得到了IVd和IVd′的混合物,其质量比大约是2∶1;当以脂肪族烯烃为底物时,也可以顺利得到相应的产物,但当底物为杂环烯烃2-乙烯基吡啶时,反应没有给出目标产物。底物拓展结果为

3、 产物表征
IVa:浅黄色固体,熔点106~108 ℃;1H NMR (500 MHz,CDCl3) δ为8.237~8.219 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H), 8.043~8.027 (d, J=8.0 Hz, 1H,Ar-H),7.861~7.828 (m, 1H,Ar-H),7.708~7.676 (m, 1H,Ar-H),7.448~7.411 (m, 2H,Ar-H), 7.291~7.175 (m, 3H,Ar-H), 5.713~5.685 (dd, J1=7.5 Hz, J2=6.5 Hz, 1H,CH), 3.776~3.743 (dd, J1=10.5 Hz, J2=6.0 Hz, 1H,CH2), 3.624~3.587 (dd, J1=10.5 Hz, J2=8.0 Hz, 1H,CH2);13C NMR (125 MHz, CDCl3) δ为151.05, 143.69, 135.12, 134.91, 132.26, 129.74, 128.62, 128.52, 128.02, 125.39, 121.95, 88.78, 3.44;ESI-HRMS m/z C15H12IN3O2[M+H]+理论值393. 0, 检测值393.0。
IVb:白色固体,熔点134~136 ℃;1H NMR (500 MHz, CDCl3) δ为8.347~8.329 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H, Ar-H), 8.155~8.139 (d, J=8.0 Hz, 1H,Ar-H), 7.966~7.933 (m, 1H,Ar-H), 7.812~7.779 (m, 1H,Ar-H), 7.679~7.662 (m, 1H,Ar-H), 7.298~7.235 (m, 2H,Ar-H), 7.161~7.147 (d, J=7.0 Hz, 1H,Ar-H), 6.138~6.109 (dd, J1=8.0 Hz, J2=6.0 Hz, 1H,CH), 3.840~3.807 (dd, J1=10.5 Hz, J2=6.5 Hz, 1H,CH2), 3.732~3.615 (dd, J1=10.0 Hz, J2=8.0 Hz, 1H,CH2), 2.444 (s, 3H,CH3);13C NMR (125 MHz, CDCl3) δ为151.25, 143.82, 137.22, 135.04, 133.72, 132.39, 130.68, 129.52, 128.75, 127.53, 126.98, 125.53, 122.06, 85.22, 19.46, 3.38;ESI-HRMS m/z C16H14IN3O2 [M+H]+理论值407.0, 检测值407.0。
IVc:白色固体,熔点117~119 ℃;1H NMR (500 MHz, CDCl3) δ为8.326~8.308 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H,Ar-H), 8.134~8.118 (d, J=8.0 Hz, 1H,Ar-H), 7.953~7.920 (m, 1H,Ar-H), 7.779~7.767 (m, 1H,Ar-H), 7.444~7.415 (m, 2H, Ar-H), 6.886~6.856 (m, 2H,Ar-H), 5.785~5.757 (dd, J1=8.0 Hz, J2=6.0 Hz, 1H,CH), 3.873~3.846 (dd, J1=10 Hz, J2=6 Hz, 1H,CH2), 3.779 (s, 3H,CH3), 3.704~3.667 (dd, J1=10.5 Hz, J2=8.0 Hz, 1H, CH2);13C NMR (125 MHz, CDCl3) δ为160.58, 151.10, 143.74, 134.91, 132.26, 129.56, 128.64, 126.99, 125.42, 121.99, 113.92, 88.62, 65.17, 3.63;ESI-HRMS m/z C16H14IN3O3 [M+H]+理论值423.0, 检测值423.0。
IVd,IVd′:浅黄色固体,熔点112~124 ℃;1H NMR (500 MHz, CDCl3) δ为8.342~8.315 (ddd, J1=8.0 Hz, J2=4.5 Hz, J3=1.0 Hz, 3H,Ar-H), 8.176~8.099 (m, 3H,Ar-H), 7.989~7.941 (m, 3H,Ar-H), 7.825~7.788 (m, 3H,Ar-H), 7.520~7.502 (m, 3H,Ar-H), 7.461~7.420 (ddd, J1=14.5Hz, J2=7.5 Hz, J3=5.0 Hz, 3H,Ar-H), 7.358~7.311 (m, 4H,Ar-H), 7.249~7.211 (t, J=8.0 Hz, 1H,Ar-H), 7.152~7.134 (m, 1H,Ar-H), 5.741~5.694 (dd, J1=8.0 Hz, J2=6.0 Hz, 2H,CH2), 5.550~5.519 (dd, J1=10.0 Hz, J2=5.5 Hz, 1H,CH), 5.131~5.089 (t, J=10.5 Hz, 1H,CH), 4.907~4.874 (dd, J1=11.0 Hz, J2=5.5 Hz, 1H,CH), 3.831~3.798 (dd, J1=10.5 Hz, J2=6.0 Hz, 2H,CH2), 3.673~3.635 (m, 2H,CH2);ESI-HRMS m/z C15H11ClIN3O2 [M+H]+理论值427.0,实测值427.0。
IVe:白色固体,熔点125~127 ℃;1H NMR (500 MHz, CDCl3) δ为8.358~8.340 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H,Ar-H), 8.160~8.145 (d, J=8.0 Hz, 1H,Ar-H), 7.977~7.915 (m, 1H,Ar-H), 7.824~7.791 (dt, J1=23.5 Hz, J2=8.0 Hz, 1H,Ar-H), 7.425~7.409 (d, J=8.0 Hz, 2H,Ar-H), 7.200~7.141 (t, J=8 Hz, 2H,Ar-H), 5.805~5.777 (dd, J1=8.0 Hz, J2=6.5 Hz, 1H,CH2), 3.887~3.853 (dd, J1=10.5 Hz, J2=6.5 Hz, 1H,CH2), 3.727~3.690 (dd, J1=10.5 Hz, J2=8.0 Hz, 1H,CH), 2.349 (s, 3H,CH3);13C NMR (125 MHz, CDCl3) δ为151.48, 144.15, 140.18, 135.27, 132.61, 132.50, 129.66, 129.03, 128.38, 125.83, 122.41, 89. 17, 21.62, 3.91;ESI-HRMS m/z C16H14IN3O2 [M+H]+理论值407.0, 实测值407.0。
IVf:白色固体,熔点125~127 ℃;1H NMR (500 MHz, CDCl3) δ为8.333~8.315 (dd, J1=8.0Hz, J2=1.0 Hz, 1H,Ar-H), 8.136~8.120 (d, J=8.0 Hz, 1H,Ar-H), 7.949~7.916 (m, 1H,Ar-H), 7.796~7.764 (m, 1H,Ar-H), 7.469~7.452 (d, J=8.5 Hz, 2H,Ar-H), 7.395~7.378 (m, 2H,Ar-H), 5.798~5.771 (t, J=7.0 Hz, 1H,CH2), 3.855~3.821 (dd, J1=10. 5Hz, J2=6.5 Hz, 1H,CH2), 3.719~3. 683 (dd, J1=10.5 Hz, J2=6.5 Hz, 1H,CH), 1.286 (s, 9H,C(CH3)3);13C NMR (125 MHz, CDCl3) δ为152.82, 143.78, 134.92, 132.27, 132.14, 128. 65, 127.71, 125.53, 125.43, 122.08, 88.74, 88.08, 34.65, 31.17, 3.66;ESI-HRMS m/z C19H20IN3O2 [M+H]+理论值449.1, 实测值449.1。
IVg:白色固体,熔点141~143 ℃;1H NMR (500 MHz, CDCl3) δ为8.289~8.271 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H,Ar-H), 8.088~8.072 (d, J=8.0 Hz, 1H,Ar-H), 7.951=7.890 (m, 1H,Ar-H), 7.784~7.741 (m, 1H,Ar-H), 7.481~7.445 (m, 2H,Ar-H), 7.336~7.293 (m, 3H,Ar-H), 5.682~5.665 (d, J=8.5 Hz, 1H,CH), 4.780~4.723 (m, 1H,CH), 2.306~2.293 (d, J=6.5 Hz, 3H,CH3);13C NMR (125 MHz, CDCl3) δ为151.01, 143.72, 135.40, 134.89, 132. 23, 129.63, 128.17, 128.65, 128.15, 125.37, 121.98, 92.52, 25.56, 24.79;ESI-HRMS m/z C16H14IN3O2 [M+H]+理论值407.0,实测值407.0。
IVh:白色固体,熔点114~116 ℃;1H NMR (500 MHz, CDCl3) δ为8.215~8.197 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H,Ar-H), 8.034~8.018 (d, J=8.0 Hz, 1H,Ar-H), 7.859~7.826 (m, 1H,Ar-H), 7.704~7.672 (m, 1H,Ar-H), 7.437~7.409 (m, 2H,Ar-H), 6.970~6.936 (t, J=8.5 Hz, 2H,Ar-H), 5.682=5.654 (dd, J1=8.0 Hz, J2=6.0 Hz, 1H,CH), 3.762~3.729 (dd, J1=10.5 Hz, J2=6.0 Hz, 1H,CH2), 3.591~3.554 (dd, J1=10.0 Hz, J2=8.0 Hz, 1H,CH2);13C NMR (125 MHz, CDCl3) δ为163.61 (d, 1JC-F=247.5 Hz), 151.33, 143.99, 135.33, 132.69, 131.40(d, 4JC-F=2.5 Hz), 130.37(d, 3JC-F=2.5 Hz), 128.99, 125.70, 122.24, 115.92 (d, 2JC-F=21.25 Hz), 88.33, 3.80;ESI-HRMS m/z C15H11FIN3O2[M+H]+理论值411.0, 实测值411.0。
IVi:浅黄色固体,熔点113~115 ℃;1H NMR (500 MHz, CDCl3) δ为8.391~8.373 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H,Ar-H), 8.221~8.205 (d, J=8.0 Hz, 1H,Ar-H), 8.004~7.971 (m, 1H,Ar-H), 7.848~7.816 (m, 1H,Ar-H), 7.676~7.661 (d, J=7.5 Hz, 1H,Ar-H), 7.438~7.407 (td, J1=7.5, J2=1.0 Hz, 1H,Ar-H), 7.364~7.306 (m, 2H,Ar-H), 6.269~6.265 (d, J=2.0 Hz, 1H,CH2), 5.083~5.062 (m, 1H,CH), 4.136~4.089 (m, 1H,CH2), 3.434 (dd, J1=14.5 Hz, J2=12.0 Hz, 1H,CH2);13C NMR (125 MHz, CDCl3) δ为151.46, 143.99, 143.17, 135.54, 135.13, 132.49, 130.84, 128.86, 127.55, 127.01, 125.59, 125.16, 122.19, 99.22, 43.87, 23.63;ESI- HRMS m/z C16H12IN3O2 [M+H]+理论值405.0,实测值405.0。
IVj:浅黄色固体,熔点83~85 ℃;1H NMR (500 MHz, CDCl3) δ为8.347~8.329 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H,Ar-H), 8.181~8.165 (d, J=8.0 Hz, 1H,Ar-H), 7.990~7.956 (m, 1H,Ar-H), 7.831~.786 (m, 1H,Ar-H), 4.754~4.723 (td, J1=6.0, J2=3.5 Hz, 1H,CH), 4.580~4.548 (dd, J1=10.0 Hz, J2=6.0 Hz, 1H,CH), 2.519~2.458 (m, 1H,CH2), 2.273~2.270 (m, 1H,CH2), 2.023~1.942 (m, 2H,CH2), 1.926~1.856 (m, 1H,CH2), 1.706~1.624 (m,1H,CH2), 1.562~1.457 (m, 2H,CH2);13C NMR (125 MHz, CDCl3) δ为151.32, 143.83, 135.01, 132.36, 128.66, 125.45, 122.06, 88.17, 34.07, 28.44, 27.24, 24.16, 21.59;ESI-HRMS m/z C13H14IN3O2 [M+H]+理论值371.0, 实测值371.0。
IVk:白色固体,熔点128~130 ℃;1H NMR (500 MHz, CDCl3) δ为8.284~8.266 (dd, J1=8.0 Hz, J2=1.0 Hz, 1H,Ar-H), 8.057~8.041 (d, J=8.0 Hz, 1H,Ar-H), 7.892 ~7.838 (m, 3H,Ar-H), 7.809~7.790 (dd, J1=8.0 Hz, J2=1.5 Hz, 2H,Ar-H), 7.726~7.695 (m, 2H,Ar-H), 7.487~7.433 (m, 2H,Ar-H), 5.980~5.952 (dd, J1=7.5 Hz, J2=6.5 Hz, 1H,CH), 3. 943~3.909 (dd, J1=10.5 Hz, J2=6.5 Hz, 1H,CH2), 3.777~3.740 (dd, J1=10.5, J2=8.0 Hz, 1H,CH2);13C NMR (125 MHz, CDCl3) δ为151.53, 144.11, 135.32, 134.25, 133.08, 133. 01, 132.67, 129.08, 129.04, 129.83, 128.62, 128.13, 127.21, 126.79, 125.80, 124.75, 122.38, 89.41, 39.50;ESI-HRMS m/z C19H14IN3O2[M+H]+理论值443.2, 实测值443.2。
4、 结 论
在反应温度为25 ℃下,以二氯乙烷为溶剂,采用摩尔比为2∶1∶2的烯烃、3-羟基-1,2,3-苯并三嗪-4(3H)-酮和NIS,通过三组分加成反应实现了烯烃的碘氧双官能团化反应,成功地合成了一系列的3-(2-碘代-1-苯基乙氧基)苯并三嗪类化合物。所有产物均经1H NMR,13C NMR确认。该反应具有条件温和、底物适用范围较好和区域选择性好等优点。同时,在查阅文献的基础上,推测该反应产物可以被锌粉等试剂还原成2-碘代苯乙醇类化合物,后续将展开相关的研究。
参考文献
[1] SAMANTA S,DONTHIRI R R,RAVICR,et al.Phenyl iodonium diacetate mediated oxidative functionalization of styrenes with molecular oxygen:synthesis of α-oxygenated ketones[J].Journal of organic chemistry,2016,81(8):3457-3463.
[2] LUO J,ZHANG J.Aerobic oxidation of olefins and lignin model compounds using photogenerated phthalimide noxyl radical[J].Journal of organic chemistry,2016,81(19):9131-9137.
[3] WANG Y,ZHANG L,YANG Y H,et al.Alkene oxyalkylation enabled by merging Rhenium catalysis with hypervalent iodine(III) reagents via decarboxylation[J].Journal of the American chemistry society,2013,135(48):18048-18051.
[4] ASHIKARI Y,SHIMIZU A,NOKAMI T,et al.Halogen and chalcogen cation pools stabilized by DMSO.Versatile reagents for alkene difunctionalization[J].Joural of the American chemistry society,2013,135(43):16070-16073.
[5] WANG Y J,WANG J X,XIONG Y,et al.I2O5-mediated bromohydroxylation and dibromination of olefins using KBr in water[J].Tetrahedron letters,2014,55(16):2734-2737.
[6] JIANG X Y,QING F L.Copper-catalyzed three-component oxytrifluoromethylation of alkenes with sodium trifluoromethane sulfinate and hydroxamic acid[J].Angewande chemie international edition,2013,52:14177-14180.
[7] LU Q Q,LIU Z L,ZHANG G H,et al.Copper-cobalt-catalyzed highly selective radical dioxygenation of alkenes[J].Organic letters,2015,17(14):3402-3405.
[8] RIBEIRO R D,ESTEVES P M,DE MATTOS M C S.Triiodoisocyanuric acid:a new and convenient reagent for regioselective coiodination of alkenes and enolethers with oxygenated nucleophiles[J].Tetrahedron letters,2007,48(49):8747-8751.
[9] PALSH P,GAYEN K S,KHAMARUI S,et al.Addition of halide to π-bond directly from aqueous NaX solution:a general strategy for installation of two different functional groups[J].Chemical communication,2011,47(24):6933-6935.
[10] SONG S ,HUANG X Q,LIANG Y F,et al.From simple organobromides or olefins to highly value-added bromohydrins:a versatile performance of dimethyl sulfoxide[J].Green chemistry,2015,17(5):2727-2731.
[11] RAO D S,REDDY T R,BABACHARY K,et al.Regioselective vicinal functionalization of unactivated alkenes with sulfonium iodate(I) reagents under metal-free conditio[J].Organic & biomolecular chemistry,2016,14(31):7529-7543.
[12] BELTRAN R,NOCQUET T S,BLANCHARD F,et al.PIFA-mediated ethoxyiodination of enamides with potassium iodide[J].Organic & biomolecular chemistry,2016,14(36):8448-8451.
[13] LI J,LI Z Q,ZHANG X,et al.Catalytic enantioselective bromohydroxylation of aryl olefins with flexible functionalities[J].Organic chemistry frontiers,2017,4(6):1084-1090.
[14] BARLUENGA J,MARCO-ARIAS M,GONZALEZ-BOBES F,et al.Reaction of alkenes with hydrogen peroxide and sodium iodide:a nonenzymatic biogenic-like approach to iodohydrins[J].Chemistry-a European journal,2004,10(7):1677-1682.
[15] BRASEY T,BURYAK A,SCOPELLITI R,et al.Synthesis of a metallamacrocyclic Re(CO)3complex using a tridentate bridging ligand[J].European journal of inorganic chemistry,2004(5):964-967.
[16] CARPINO L A,XIA J S,ZHANG C W,et al.Organophosphorus and nitro-substituted sulfonate esters of 1-hydroxy-7-azabenzotriazole as highly efficient fast-acting peptide coupling reagents[J].Journal of organic chemistry,2004,69(1):62-71.
[17] ANDERSEN P,ANKERSEN M,JESSEN C U,et al.The synthesis of a GH-releasing compound NNC 26-1089[J].Organic process research & development,2002,6(4):367-373.
[18] BAG R,SAR D,PUNNIYAMURTHY T.Iron(III)-catalyzed aerobic dioxygenation of styrenes using N-hydroxyphthalimide and N-hydroxybenzotriazole[J].Organic & biomolecular chemistry,2016,14(12):3246-3255.





